August 14, 2019
E-IRB Protocol History & Application Attachments [HTML]
The E-IRB system retains a Protocol History that enables future users to retrieve relevant IRB application materials throughout the life of the study. Continue reading for an explanation of what Protocol History entails and the established standards for retaining attachments.
In Protocol History under the Protocol Approvals tab, there is a “View Attachments” link that provides a list of attachments available for each ‘iteration’ of the application (1st ‘iteration’ is Initial Review (IR); 2nd‘iteration’ may be a Modification Request (MR) or Continuation Review (CR), and so on).
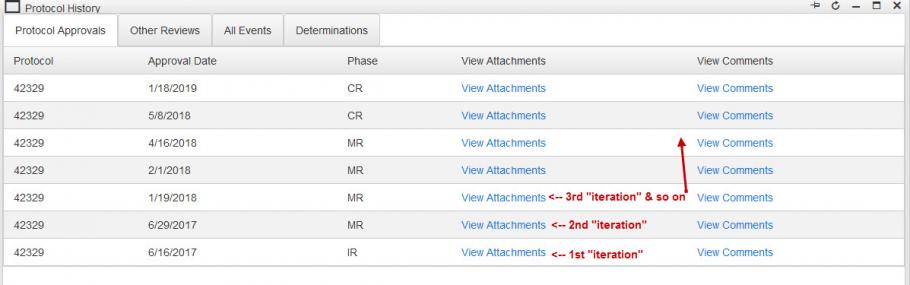
The “View Attachments” feature functions as a snapshot of the approved application as it existed when approval was granted (be it IR, CR, MR, etc.), so each of those iterations needs to represent the entire protocol as it should be implemented per IRB approval.
When a new iteration is created (e.g., draft MR, CR), E-IRB automatically carries forward all attachments from the previous iteration, and allows the researcher to remove those that are outdated or obsolete.
An outdated document could be one that has been replaced by a revised document (e.g., a data collection tool that is no longer going to be used because a revised one was created to acquire additional data), or that represented a component of the research that no longer is being conducted (e.g., a study is closed to enrollment, so advertising materials used for recruitment are no longer needed).
It is the responsibility of the researcher to maintain the most current attachments with each iteration (the E-IRB system will never delete attachments of its own accord).
Examples:
- If you create an MR to revise one of your two IRB-approved informed consent/assent documents, in the Informed Consent Section attachments you will see a list of the ‘clean’ copies of the two previously approved informed consent/assent documents. You will need to remove the old version of the informed consent form (ICF) you wish to revise to prevent it from being stamped. The ICF that is not being revised can be left alone. Upload a tracked-changes (“highlighted”) copy of the revised ICF assigned the Document Type “Highlighted Changes” and an untracked (‘clean’) copy of the revised ICF assigned the applicable Document Type (e.g., Informed consent/HIPAA Combined Form) for IRB review. Upon approval of your MR only the unchanged ICF and the clean copy of the revised ICF will get an “IRB Approval” stamp. In Protocol History, the “View Attachments” link for this MR will display a list of attachments including the newly approved & stamped revised ICF and all previously approved attachments minus the old version of the ICF you removed.
- If you have a study that involves off-site research and included a letter of support at Initial Review to get your study approved, when you create a CR, that letter of support will automatically be included in the attachments. If the research is continuing to be conducted at that off-site facility, you would leave the letter of support alone (do not remove it). When approval for CR is issued, the View Attachments link in Protocol History for this CR will include the off-site letter, which appropriately reflects that approval for that off-site facility has been maintained.
Instructions about deleting files/attachments are in the Informed Consent section (see highlighted text below in screen shot), and in E-IRB Video Tutorials on ‘Adding Informed Consent Section Attachments’ (~ minute 4:00), and ‘Responding to Requested Revisions’ (~ minute 5:40).

If you have inadvertently acquired IRB approval for an outdated/obsolete document, it is recommended that a Modification Request be created & submitted to correct the records.