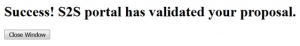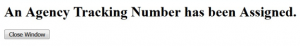Click here for Cayuse Submission by PI Detailed Instructions (pdf).
Electronic Resources
Access to Electronic Systems
Many research funding sponsors have their own electronic system for proposal submission and/or post-award management. Some of these systems allow for PIs to register directly, while other systems require institutional registration or affiliation, handled by OSPA. The University of Kentucky maintains accounts with all major federal sponsors as well as the most popular foundation and for-profit organizations.
For creation of an eRA Commons account, affiliation of your existing eRA Commons or NSF, https://www.research.gov/, with UK, or if you have questions about a new electronic system, send an email to ospa@uky.edu.
ORCID ID and eRA Commons
Per NOT-OD-19-109, NIH, AHRQ, and CDC announce that individuals supported by research training, fellowship, research education, and career development awards will be required to have ORCID iDs (Open Researcher and Contributor Identifiers) connected to their eRA Commons account beginning in FY 2020.
Instructions to connect your ORCID ID with your eRA Commons account are available here.
In October 2019, the requirement for ORCID identifiers will be incorporated into the appointment process for trainees, scholars, and participants supported by institutional research training, career development, and research education awards that require appointments through the xTrain system, including the following:
T03, T15, T32, T34, T35, T37, T42, T90/R90, TL1, TL4, TU2, K12/KL2, R25, R38, RL5, RL9
At the time of appointment, the xTrain system will check whether appointees have ORCID iDs and appointments will not be accepted for agency review unless an ORCID iD is linked to the individual's eRA Commons Personal Profile.
Beginning with receipt dates on or after January 25, 2020, NIH has instituted a requirement for ORCID identifiers that will be enforced at the time of application for individual fellowship and career development awards, including the following:
F05, F30, F31, F32, F33, F37, F38, F99/K00, FI2, K01, K02, K05, K07, K08, K18, K22, K23, K24, K25, K26, K38, K43, K76, K99/R00
eRA system validations will check whether applicants have ORCID iDs and applications will not be accepted unless an ORCID iD is linked to the PD/PI's eRA Commons Personal Profile.
Internal Approval Form (IAF)
The IAF must be received by OSPA at least three business days prior to the sponsor’s published deadline. If it doesn’t meet the deadline, the proposal is prioritized AFTER proposals which did meet the 3 day deadline.
- Example of three business days: If the sponsor has a deadline for electronic proposal submission of 5:00 p.m. on Thursday, May 10 the completed IAF is due in OSPA before 9:00 a.m. on Tuesday, May 8.
- April 2 is a Monday. If the sponsor has a deadline for electronic proposal submission of 5:00 p.m. on April 2, a completed IAF must be received by OSPA before 9:00 a.m. on Thursday, March 29.
- A fully executed IAF means that the PI, all co-Investigators, applicable chairs, directors and deans or associate deans have certified electronically through workflow in MyUK. Once the final certification has been made, the PI and OSPA will be notified by email.
- Instructions for completing the IAF through MyUK (pdf, 10pgs). Contact OSPA for additional help.
- Instructions for selecting an IAF delegate (pdf, 3 pgs).
All investigators must have completed the on-line financial disclosure form before a proposal is submitted. In addition, a new account will not be established until all investigators have completed the disclosure and taken the Conflict of Interest Education Course.
Cayuse Proposals (S2S) Preparation & Submission System
UK researchers will now have access to a new system that will make it easier to create fully developed, compliant and accurate proposals for submission to federal and non-federal sponsors. The Cayuse Proposals (S2S) Preparation and Submission system (formerly Cayuse 424) is designed to provide faculty and staff with a mechanism to streamline the proposal process by storing and auto-populating institutional information, budgetary calculations, and uploadable profiles for Principal Investigators. There is real-time validation that is provided in easy-to-understand language and prompts for correction to ensure compliance the first time. The entire system is designed to make the application process less of a burden for researchers - a crucial part of ensuring quality and quantity of proposals generated.
OSPA will be providing training and front-line support for the system. There will be a training period with a variety of offerings available. Check below for training opportunities. No registration is required.
- Cayuse Proposals (S2S) FAQs
- Access the Cayuse Proposals (S2S) User Manual (pdf, 145 pgs)
- Access the Cayuse Proposals (S2S) System - Use your LinkBlue ID and password
More Information
A video presentation by Kim Carter on the new system is available on the Video Library. A LinkBlue login is required.
Both Introductory Presentations and Computer Lab sessions will be scheduled as needed for training. Visit the OSPA Events calendar to check for an available session or contact OSPA to schedule one.
Cayuse Submission by PI FAQs
What application types are eligible for PI submit?
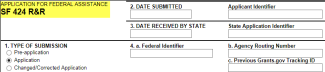
Grants.gov SF424 R&R application types will be eligible for PI submit, except:
Applications for Federal Assistance SF424 that meet the criteria below will not be eligible for PI submit.
- All application components are uploaded as one complete packet. A separate field is not identified for uploading the budget justification.
- All application components are uploaded as separate documents utilizing the ‘other attachments’ form page. A separate field is not identified for uploading the budget justification.
What are the Errors, Warnings, and Info notices I am seeing in Cayuse?
The Validation panel in Cayuse Proposals (S2S) shows three different types of messages: Errors, Warnings and Info. Here’s a summary of what each means:
- Errors in Cayuse Proposals (S2S) reflect problems that will impose a hard-stop rejection at Grants.gov or the funding agency. Agency errors display the agency name in brackets to the right of the field in question (e.g., [Research Plan 2.2][NIH]). To ensure that your submission is successful, Cayuse Proposals (S2S) runs a final check on Grants.gov and agency validations before you submit a proposal. Actual agency validations may vary depending on the federal opportunity instructions. When it comes to agency-specific errors, the current federal opportunity instructions supersede Cayuse error messages.
- Cayuse errors (e.g., [SF424A][Cayuse]) display ‘Cayuse’ is brackets. This is an error that is created specifically for Cayuse, not in response to any outside validations. Even with this error/warning, the Proposal can be submitted. The instructions for the Proposal supersede the Error that Cayuse is giving. To be absolutely clear, follow the instructions provided by the Opportunity, not this specific error in Cayuse.
- Warnings pertain to agency validations or may serve as notices from Cayuse regarding missing attachments. For example, Cayuse Proposals (S2S) does not require a budget justification; however, Cayuse 424 displays a Warning, because a budget justification is a common attachment. Warnings do not prevent proposals from being submitted or from being reviewed.
- Info notices are Cayuse recommendations. Forgetting to enter a Sponsor Deadline, for example, prevents the Deadline from appearing in your list of proposals. Cayuse Proposals (S2S) will alert you when this happens. Info messages are specific to the software and do not prevent proposals from being submitted or from being reviewed.
Error – Other Attachments on HRSA proposals
We've had several other inquiries about this [HRSA] agency-specific error that states "The Other Attachments are not used by HRSA". The opportunity requires certain attachments and, as observed, there just isn't anywhere else to put them besides the "Other Attachments" section. Because of this, we recommend using the "Other Attachments" section despite the error.
The agency-level error described above won't prevent electronic submission to Grants.gov. Using the Other Attachments section does go against HRSA's standard validations, but our understanding is that this opportunity allows for this exception. We also know that one of the institutions we work with submitted with attachments in the Other Attachments section without a problem, so we don't anticipate you'll have submission troubles.
Will my RA or CGO be available to assist me if I experience problems with my submission after 5pm or over the weekend?
OSPA is available to provide assistance with submissions Monday-Friday from 8am-5pm. OSPA encourages submission of proposals well in advance of agency deadlines so any issues can be caught and addressed without impacting submission. While some agencies may have submission deadlines after 5pm, the OSPA office closes at 5pm.
How will the administrative lockdown affect the ‘PHS Human Subjects and Clinical Trials Information’ section?
Please note that ‘PHS Human Subjects and Clinical Trials Information’ will need to be completed prior to the PI approval of the application as this is considered part of the application components.
The administrative lockdown will affect the following:
- The investigator will be unable to edit fields in existing studies already entered in the proposal.
- The investigator will be able to add a new study record (manual entry and PDF import) to the proposal but will be unable to edit the data fields after manual creation of the study record and post-import of the PDF.
The administrative lockdown will not affect the following:
- The investigator will be able to add/delete attachments on existing studies already entered in the proposal.
- The investigator will be able to delete a study record already entered in the proposal.
The check-off box is highlighted for me, but when I try to click to “check off and approve” the proposal nothing happens, and my approval does not register in the routing chain. What should I do?
This is caused by a browser conflict. Check your browser to make sure you are using one of the compatible browsers. https://support.cayuse.com/hc/en-us/articles/115013529288-Browser-Support-and-Configuration
I am next in the routing chain but the box is grayed out so I cannot “check off and approve” my proposal. What should I do?
When your user account was created it may not have been linked to your professional profile. Please contact your OSPA CGO to correct.
I start Cayuse, open my proposal and the screen is mostly white, content is missing, and I can’t do anything within the forms. What should I do?
This is a browser cache issue. The Cayuse software may have been upgraded since you last used Cayuse, but your browser is still trying to draw from your history. Clear your browser cache and this should correct the problem.
I get an error when I upload a pdf. How do I fix this?
Cayuse cannot accept fillable or encrypted PDFs. The solution is to print, scan, and then upload the "flattened" PDF.
I can't upload an Excel attachment, and the sponsor requires it. What do I do?
Prior to the administrative lockdown of the proposal contact your CGO who will have a system administrator upload it.
When I print the pdf of the proposal file things aren't in the order I expected. What's wrong?
The order of the Cayuse pdf doesn't necessarily reflect the order of the pages as received by the sponsor's system. Also Excel documents will not print in the Cayuse generated pdf. For NIH, view the proposal in eRA Commons where you'll see the final version.
Cayuse Submission by PI Detailed Instructions
Review the application package for eligibility.
SF 424 R&R application form will be eligible for PI submit.
Applications for Federal Assistance SF-424 that meet the criteria below will not be eligible for PI submit:
- All application components are uploaded as one complete packet. A separate field is not identified for uploading the budget justification.
- All application components are uploaded as separate documents utilizing the ‘other attachments’ form page. A separate field is not identified for uploading the budget justification.
Routing the Proposal to OSPA for Review/Approval
Cayuse features a ROUTING CHAIN, which provides additional flexibility by enabling either OSPA or the PI to ultimately submit the final proposal.
Use of the routing chain enables the PI to route “administrative” portions of the proposal (data fields and budget related attachments) to OSPA for review, while still continuing to work on the “scientific” portions (project narrative, biosketches, etc.) over the last days leading up to the deadline. OSPA can then approve the proposal for submission within the routing chain, which will ultimately allow the PI to submit the final proposal to the sponsor at their convenience.
Prior to routing, be sure that all form data is entered, and the budget justification is uploaded, as these portions “lock down” and can no longer be edited after routing.
Cayuse Administrative Lockdown
Administrative Lockdown is not an actual feature per se in Cayuse; it is, however, what occurs when the PI approves the application under the routing and approval section. This action by the PI locks down the application components, the budget and budget justification. The PI will still be able to attach the project narrative and other required documents.
Please note that the ‘PHS Human Subjects and Clinical Trials Information’ will need to be completed prior to the PI approval of the application as this is considered part of the application components.
We should note that there are some applications that are not eligible for PI submission. These consist of a single attachment that contains the budget, budget justification and all required documents as one uploaded file.
To Route the Proposal
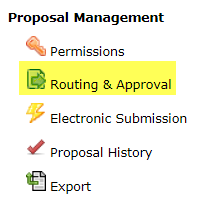
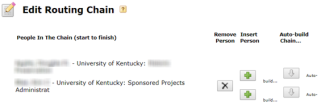
Click the “Routing & Approval” link. Check the routing chain and edit as needed; it must always begin with the PI and end with your OSPA Research Administrator. You must inform your Research Administrator that a proposal is coming to allow sufficient time for review.
Once the routing chain is initiated, OSPA will retrieve and review the proposal, work with the investigator through any corrections needed and will ultimately approve the proposal for submission. At that point, any remaining file attachments can be uploaded, and the proposal can be submitted at any time up until the deadline.
Keep in mind that OSPA staff is available to submit during normal business hours only (M-F 8am-5pm), even though deadlines sometimes fall later in the evening or overnight. In such cases, investigators should plan to submit themselves if the proposal will not be finalized by close of business on the deadline day.
Submitting the Final Proposal
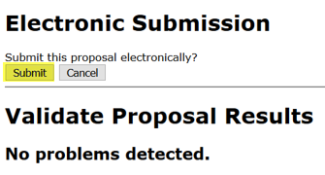
To submit, click the “Electronic Submission” icon. It is highly recommended to “Validate Proposal” prior to submission, which runs one final check for errors. If any new errors are identified here, these should be resolved before proceeding with submission. “Errors” are usually terminal and must be corrected before a proposal can be successfully submitted. “Warnings” are potential problems that may or may not require attention. “Info” are items that Cayuse interprets as a potential issue, but do not necessarily link to a programmed system validation.
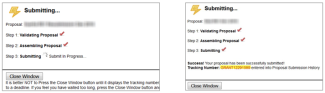
After clicking “Submit to Grants.gov,” a pop-up window will appear and show the progress as the proposal is validated, assembled, and transmitted to the grants.gov server.
Ideally, the screen will deliver a “Success!” message and a grants.gov tracking number. The entire submission process may take anywhere from a few seconds to several minutes; please be patient.
Guide to Cayuse Submission by PI
Beginning October 8, 2019, a complete and final proposal, accompanied by a fully routed Internal Approval Form (IAF), must be received by the Research Administrator (RA) in OSPA by 9:00 am at least three (3) business days prior to the sponsor’s deadline. For more information about the 3-day Proposal deadline click here.
Click here for Cayuse Submission by PI Detailed Instructions (pdf).
Click here for Guide to Cayuse Submission by PI (pdf).
Step 1: Prior to Submission
Keeping in mind the 3-Day Proposal Deadline, work with your College Grant Officer (CGO) or Grant Proposal Specialist (GPS) to ensure that your proposal is eligible for PI submission. For more information about the 3-Day Proposal Deadline click here.
- Proposals using the SF 424 R&R form are eligible for submission by PI.
- Proposals using the Application for Federal Assistance SF-424 form that meet the criteria below are not eligible for submission by PI:
- A separate field is not identified for uploading the budget justification.
- All application components are uploaded as one complete package.
- All application components are uploaded as separate documents utilizing the ‘Other Attachments’ form.
Step 2: Prepare your Proposal
Keeping in mind the 3-Day Proposal Deadline, work with your College Grant Officer (CGO) or Grant Proposal Specialist (GPS) to prepare your proposal and ensure that all administrative fields are free of errors. For more information about the 3-Day Proposal Deadline click here.
Step 3: Proposal Routing & Approval
Once your proposal is free of errors, a “routing chain” will be established. The OSPA Research Administrator for your area must approve the proposal before you can submit. If routing hasn’t been created, then:
- Select the “Routing & Approval” link.
- Check the routing chain and edit as needed; it must always begin with the PI and end with your OSPA Research Administrator.
Step 4: Submitting
Important Note: Submission requires that the browser used accepts pop-ups from Cayuse. Please ensure that any pop-up blockers are set accordingly.
When ready to submit, do the following:
- On the left-hand side navigation pane scroll down to Proposal Management and select the lightning bolt icon that says Electronic Submission. This will take you to the submission screen.
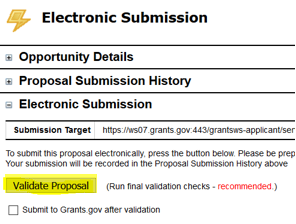
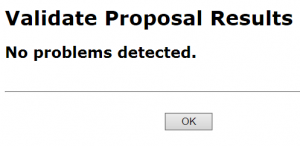
- In the Electronic Submission screen click the Validate Proposal button to check for errors one last time. You should get the message that there are no errors. If you do receive an error message you will need to correct the error before you can submit.
A pop-up screen will appear with validation results.
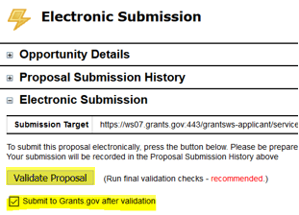
- Then check the box next to Submit to Grants.gov after Validation and click the Validate Proposal button again.
- A pop-up screen will appear for submission. Click Submit.
- The following submission steps will appear.
Wait until you get your tracking number before closing the screen. The tracking number will be in the format of GRANT0012345.
- Close the pop-up screen and click the + sign next to Proposal Submission History. You will see your tracking number as a blue link.
You can click on it to check the status of transmission and you may want to do that intermittently to make sure that the application is validated.
- Validation message #1: Validation by Grants.gov
- Validation message #2: Agency Retrieval
The agency retrieval is generally the final message and means the proposal was successfully submitted.
For NIH submissions, an "Agency Tracking Number Assignment" email should be received. Be sure to check your proposal in eRA Commons.
Times for receipt of the messages and agency retrieval vary. Messages may take minutes or hours and occasionally part of a day.
Ready to submit? Log-in to Cayuse at https://uky.cayuse424.com/