E-IRB News 2023

November 9, 2023
The following announcement, issued November 8, 2023, describes changes that were scheduled to go into effect as of the afternoon of November 9, 2023 [HTML]:
Project Information Section

Tracked Changes - Study Personnel Changes
NON-UK Personnel Not Allowed to Serve as PIIt is University of Kentucky policy that non-UK personnel cannot serve as Principal Investigator on an IRB application (they can be co-investigator, or other personnel). Therefore, individuals with an “External” status flag in the UK Human Resources personnel database will not be able to be inserted as PI on an application, so their record will not be find-able in the “Change Principal Investigator” search table.
Researcher DashboardThe researcher’s E-IRB Inbox will reflect a few column changes to improve the information provided at a glance. This includes, but is not limited to, the addition of a ‘Submit Date’ column under the “Applications” tab (this reflects the date the current phase of the application was first submitted) and removal of the ‘Edit Status’ column.
E-IRB Video Tutorial LibraryResources previously stored on MS Stream, like the E-IRB Video Tutorial Library, have been (or are being) migrated to SharePoint. For ease of viewing, a web page with a table of contents has been created for the various online video tutorials. If you have an old bookmark for the E-IRB Video Tutorial Library that points to the MS Stream site, please update it to: https://www.research.uky.edu/office-research-integrity/e-irb-video-tutorial-library. Links to the new Library web page throughout E-IRB and on ORI’s web pages will be updated accordingly. |
April 3, 2023
Key Take-Aways from March 24, 2023 E-IRB system Update [HTML]
|
For researchers, the key take-aways from the E-IRB system update that occurred on March 24, 2023 are summarized below: Deviation/Exception - Single Use Informed Consent Form (ICF)A new ‘document type’ (DocType) has been added to the options for uploading in the Deviation/Exception Request Form called “Single-Use ICF”. 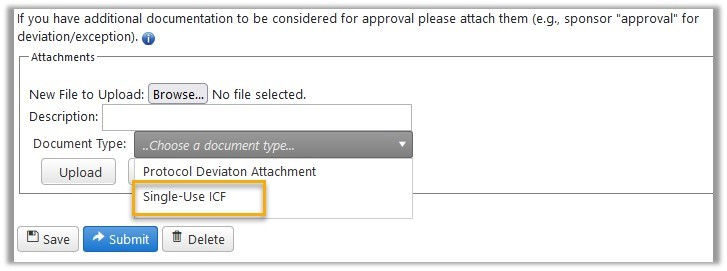 When this DocType is assigned to an attachment, upon approval of the Deviation or Exception, the informed consent form (ICF) gets a stamp with the words “Single Use” in it.  The stamped ICF is included in the file that opens when the user clicks the “View # ___” link for the applicable Deviation/Exception under the Completed Other Reviews tab in Protocol History. New AttachmentsThe following improvement was made for consistency in reflecting when an edit has been made:
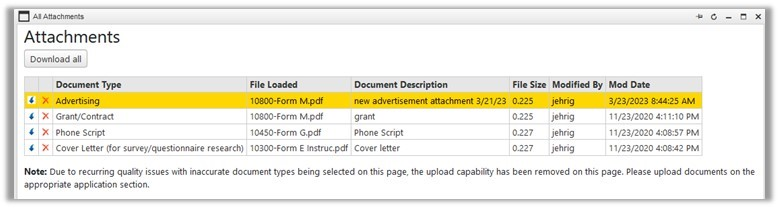
All Attachments and 'Download All' Tool“All Attachments” is the recommended place to view materials to be considered for IRB review/approval in an “In-Progress” application. Researchers should keep this in mind when tasked with removal of outdated documents as the documents may not appear within the section itself. For example: 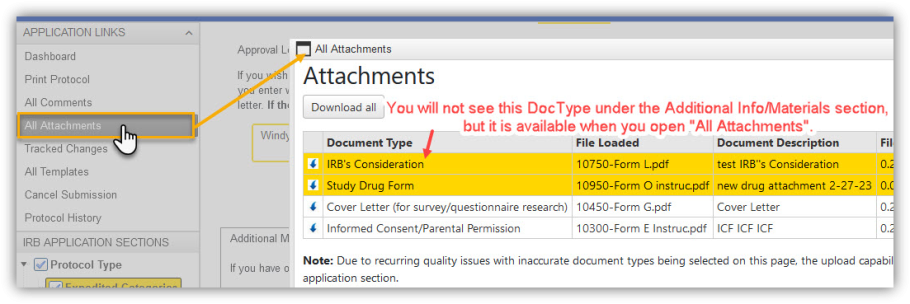 The ‘Download All’ tool now also generates a .csv file listing all the attachments in the zip folder with the following info:
As always, we hope the latest update improves your overall E-IRB experience! |
January 26, 2023
Redistributed February 8, 2023
Upcoming Changes Affecting E-IRB Navigation and Displays [HTML]
|
The E-IRB System update coming in early February involves several major changes that will impact your E-IRB experience as follows: Reorganization of Info in Application’s Blue Banner Your application’s banner will still contain all the same pertinent information about your application; the information has just been re-organized, and the tools for printing the application and returning to your Dashboard have been shifted to the Application Links menu as shown in the next section: 
How to Print Your Application and Return to the Dashboard The tools for returning to your home Dashboard and for printing your application to PDF will be the first two menu options located under the Application Links menu on the left of an application that is pending review/approval:
Currently approved applications will not have the Print Protocol menu option because the ProtocolPDF is readily available for opening on the default landing page:
How to Create a new Modification Request (MR) or Continuation Review (CR) The menu options for creating a new MR or CR will be housed under the currently approved application when the application is eligible for one or both of these application phases. For example, to create an MR for an application that doesn’t already have an MR, CR, or study closure request created, you will need to find your application under the Approved folder first, open it, (Steps 1 and 2 in below screen shot), use the Modification Request menu option under the “Create” bar (Step 3 in second screen shot), then follow the prompts for completing the MR:
The Create MR and CR/Study Closure menu options will only be available if no other phases of your application have already been created (e.g., an other MR, a CR/AAR, or Administrative Study Closure (‘Other Review’)). For example, if you have already created a CR, you will not have the menu option to start another phase -- the CR will need to get reviewed and approved before that study is eligible for a new CR or another MR to be created, and vice versa. Applications certified as exempt do not undergo Continuation Review so that menu option will not be available for them. If someone has already created a DRAFT MR, CR/AAR, or Study Closure and you are personnel with edit authorization, you can open the MR or CR/AAR and cancel that draft using the "Cancel Submission" option under the APPLICATION LINKS menu, or open the draft Administrative Study Closure from under the ‘Other Review’ tab and click the Delete button at the bottom of the form. These actions will not delete any previously approved version of your application.
How to Create an ‘Other Review’ The menu options for creating a new Deviation/Exception, Protocol Violation, and Unanticipated Problem will be housed under the currently approved application. Find your currently approved application as instructed above in “How to Create a New Modification Request (MR) or Continuation Review (CR)”. Then select the applicable ‘Other Review’ and follow the prompts to complete the submission.
Note that there is an option to create an Unanticipated Problem Report for Inactive applications, as well as new submitted IR applications (not yet approved). Open your application from under the applicable folder (‘Inactive’, ‘Submitted’, or ‘Inbox’) to view the menu options on the left-hand side:
Edit-Indicator Color Change The update also involves a change in how the system indicates an edit has been made to an application. Currently, when a change has been made to a previously-submitted application, the section title text turns red, and the affected question is outlined in red. Once the system update has been implemented, a gold background for the section title and a gold outline for the affected question will indicate changes, as shown in the following sample screen shot.
Based on user polls and other feedback received, we expect these changes to be more intuitive, and to minimize erroneously created application phases and 'Other Reviews'. Most of the E-IRB Video Tutorials will need to be updated to reflect these changes and that will take some time, so please be patient while that task is underway and feel free to share the news with your co-workers. |









