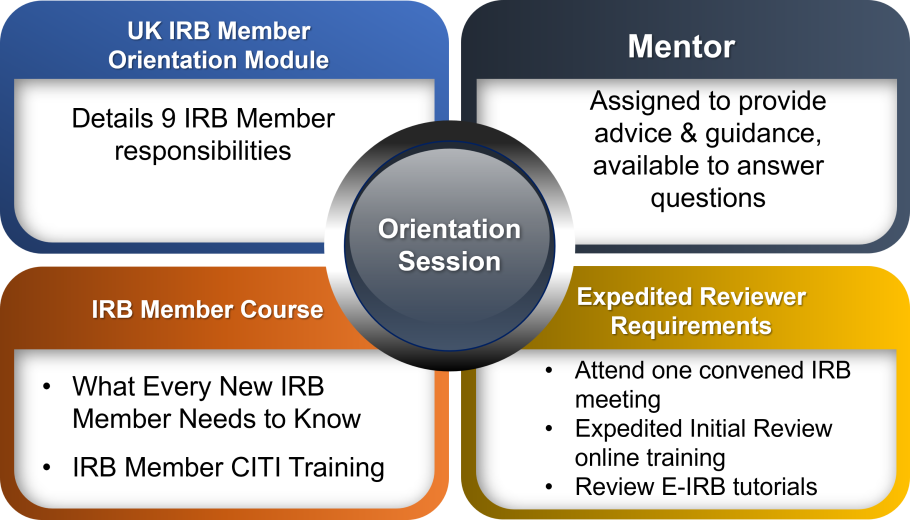
IRB Member Orientation
Overview
What We Do [Interactive Tool] [PDF]
|
New Researcher FAQs regarding the UK IRB [Interactive Tool][PDF] |
IRB Acronyms & Abbreviations [PDF]
|
Training
New IRB Member Training TO DOs
|
UK IRB Member Orientation & Quiz [PDF] |
CITI What Every IRB Member Needs to Know Instructions [PDF] CITI Community Member Training Instructions [PDF] |
Upcoming Training Events [HTML]
|
Other Training
Expedited Initial Review Training [HTML] Tips for Conducting Expedited Review [PDF] Category 5 - What's New [MP4] |
IRB Member: E-IRB Basics [HTML] The SharePoint site containing this video may prompt you to log-in using your link blue ID and password. |
E-IRB Tutorial: Primary Reviewer [HTML] The SharePoint site containing this video may prompt you to log-in using your link blue ID and password. |
Extra Information
IRB Member Q&A Guide [PDF]
 |
UK ORI Interactive FDA Flow Chart: Drugs/Biologics [HTML Interactive Tool] |
UK ORI Interactive FDA Flow Chart: Medical Device Trials [HTML Interactive Tool] |
IRB Member Roles [YouTube Video]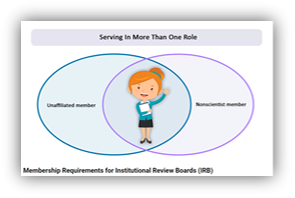 |